Apple Gets FDA Authorization for AirPods Pro 2 Hearing Aid Feature
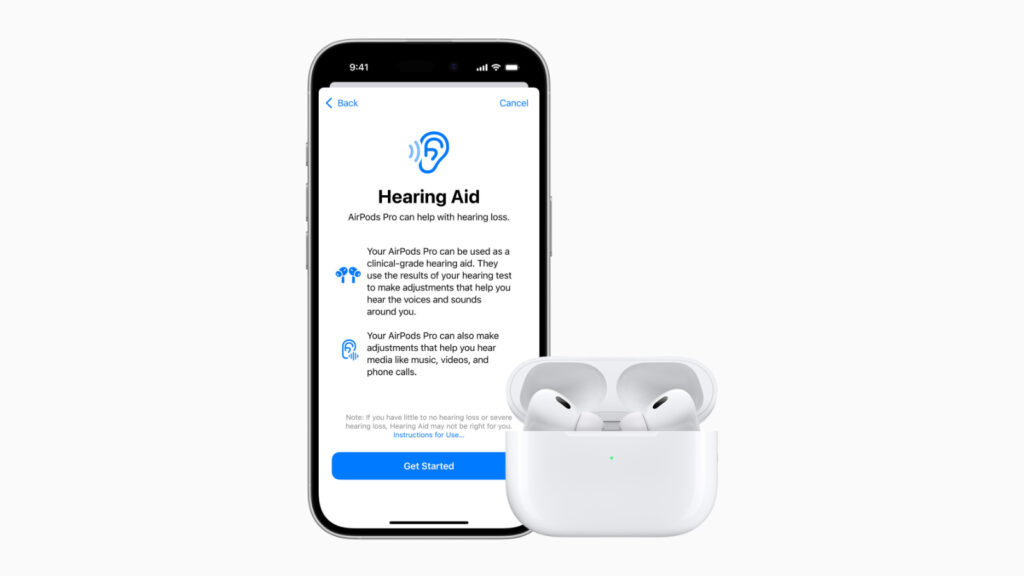
Apple will soon allow the AirPods Pro 2 to serve as an over-the-counter hearing aid, and the company has now received FDA approval for the feature.
As noted by TechCrunch, the FDA today announced that it has authorized Apple to add OTC hearing aid functionality to the AirPods Pro 2, and it marks the first product that the FDA has allowed to serve as an over-the-counter hearing aid software device.
An update coming to the AirPods Pro 2 will add the Hearing Aid functionality. Users will take a short Hearing Test using the AirPods and an iPhone, and if hearing loss is detected, a personalized sound profile is created that allows the AirPods Pro 2 to make dynamic sound adjustments to increase sound as needed.
Apple says that the clinical-grade hearing aid feature will help people better engage in conversation and stay connected to the people and environment around them.
Personalized hearing profiles are automatically applied to music, movies, games, and phone calls without the need to adjust any settings. For those with little to no hearing loss, the Hearing Test will also help with specific adjustments at individual frequencies.
Apple plans to roll out the Hearing Aid feature for AirPods Pro 2 this fall.
This article, “Apple Gets FDA Authorization for AirPods Pro 2 Hearing Aid Feature” first appeared on MacRumors.com
Discuss this article in our forums